 |
ScientiaCME
 |
Critical Care Advisor's Blog
Blog # 2 - June 2011, Dr. McGee on Hemodynamics - William T. McGee, M.D., M.H.A.
I will continue with the discussion of the use of dynamic variables of volume responsiveness at the bedside. I am not certain that we will get to the topic of oliguria today, but as we move forward in this discussion, and you understand that the utilization of dynamic parameters of volume responsiveness allow us to optimize blood flow, and also to ascertain, using our clinical knowledge of the patient, the adequacy of that flow, at least indirectly, addresses the issue of oliguria. Be assured there will be more on this topic to follow. Before I get ahead of myself, there are some significant limitations to the use of dynamic parameters in assessing volume responsiveness, and furthermore, I wanted to clarify the use of pharmacotherapy for augmentation of cardiac performance as mentioned in my initial blog.
The concept of how stroke volume variation changes and actually increases related to the volume status and volume responsiveness of the patient is critically important for the intelligent application of this parameter in clinical medicine. In terms of nomenclature, in reading the literature around dynamic variables of volume responsiveness, surrogates for stroke volume variation, namely pulse pressure variation or even systolic pressure variation, will commonly be found. Understanding the physiology that relates the variability in stroke volume (which ultimately determines blood pressure) to the degree of pre-load responsiveness is critically important for the intelligent application of these parameters at the bedside. The concept reflects basic, but perhaps not simple, physiology that we recall from our training and have witnessed at the bedside of patients throughout our careers. It is important to recognize that this discussion describes patients receiving positive pressure ventilation. The reasons for this will become apparent.
Pulsus paradoxus is a particular well known example of the interaction between the heart and lungs during spontaneous breathing seen with either wide swings in pleural pressure, a patient with status asthmaticus with excellent respiratory muscle reserve being an example of this, or cardiac tamponade related to inadequate filling of first the right and subsequently the left side of the heart related to external compression of the cardiac chambers. We recognize that pulsus paradoxus is exaggerated by hypovolemia. The physiology that I will now describe has sometimes been referred to as reverse pulsus paradoxus because it occurs during the opposite phase of the ventilatory cycle from pulsus paradoxus in patients who are being ventilated with positive pressure. Figure 1 illustrates the physiology for stroke volume variation during positive pressure ventilation.
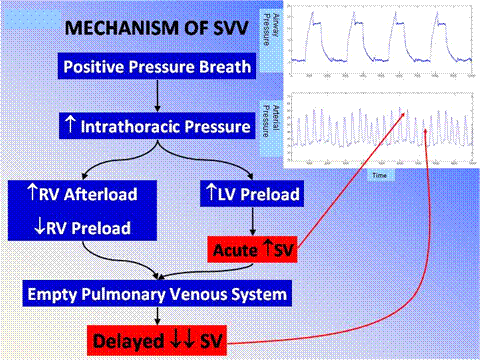
Figure 1
In Figure 1, we see displayed in the top panel the airway pressure from four positive pressure breaths simultaneously displayed with the arterial waveform directly below it. It is easy to follow the swings in blood pressure and their timing to the ventilatory cycle by looking at these two tracings together. Blood pressure goes up during the inspiratory phase of mechanical ventilation and decreases during expiration. When we see this at the bedside in a patient who is hypotensive, we know intuitively that they will respond to volume. Interestingly and importantly, those patients who don’t respond to volume actually inform us about their peculiar physiology and may lead to specific diagnoses, i.e. cardiac tamponade (more on this later).
As the ventilator cycles, there is an acute increase in intrathoracic pressure. We are familiar primarily with the dramatic impact that this may have on right ventricular filling and function. This also sets the stage for the swings in stroke volume and consequently blood pressure that we are familiar with during positive pressure ventilation. RV afterload is increased simply by the mechanical pressure of the individual alveoli on the pulmonary capillaries. The right ventricle sees that as increased afterload (Figure 2).
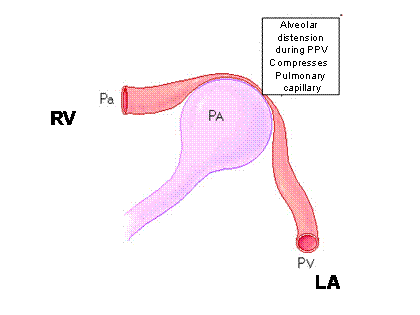
Figure 2 shows the impact of alveolar inflation on RV afterload and LA filling
RV – right ventricle; LA – left atrium; Pa – pulmonary arteriole; Pv – pulmonary vein; PA Alveolus
Simultaneously RV preload is decreased due to a smaller gradient for venous return when the chest is pressurized. Simply, the driving force for venous return (which equals cardiac output) can be simply defined as the pressure difference between intrathoracic and extrathoracic vessels. As the intrathoracic pressure increases, this pressure gradient for venous return is decreased, and therefore less blood will flow into the right side of the heart during the inspiratory phase of positive pressure ventilation. Approximately 3-5 cardiac cycles later, this smaller volume ejected by the right ventricle shows up on the left side of the heart and then in the systemic circulation as decreased stroke volume and cardiac output. This is the predominant effect of positive pressure ventilation on both stroke volume and blood pressure and is well known. Perhaps less well appreciated is the acute augmentation in stroke volume during the inspiratory phase of positive pressure ventilation. This affect at the alveolar capillary interface can simply be thought of as the alveolus inflating, compressing the adjacent pulmonary vein, and that displaced blood volume moving to the left side of the heart (Figure 2).
This acute augmentation in left ventricular preload, the right side of the diagram in Figure 1, causes an acute increase in stroke volume and consequently blood pressure during the inspiratory phase of positive pressure ventilation. This describes the basic, but perhaps not simple, physiology.
In studying Figure 1, it should become apparent that changes in the breathing pattern or in pleural pressure on a breath-to-breath basis would impact stroke volume variation dramatically. Similarly, changes in the RR interval seen on EKG or felt in the pulse would also dramatically impact stroke volume variation independent of the physiology I have just described. Furthermore, it is important to recognize, and I think we intuitively understand, that stroke volume variation is significantly impacted by the volume status of the patient. Thus, the linear relationship between stroke volume variation and preload responsiveness. The greater the stroke volume variation, the greater the response to a volume challenge. (Figure 3)
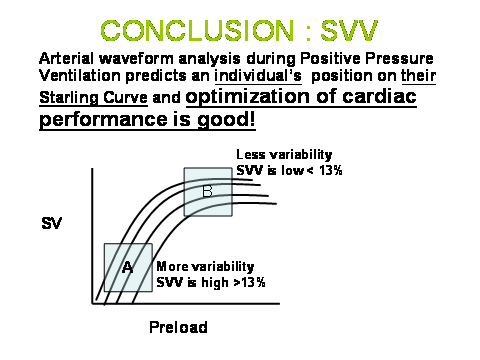
Figure 3: Multiple Starling curves represent individual patients or a single patient whose cardiac performance varies throughout the course of illness and treatment. Determining where a patient is on their own Starling curve at any point in their illness is what makes this data pair SVV:SV a major breakthrough in our ability to care for the critically ill. When SVV is elevated (>13% - A), patients are on the preload dependent part of the curve and will respond to volume. B represents the preload independent portion of he curve and if cardiac performance (SV) is inadequate, therapy other than volume is necessary to augment cardiac performance.
If filling of the ventricles is changing on a beat-to-beat interval because of a significant dysrhythmia, and atrial fibrillation is the best example of this, there is significant variation in stroke volume simply related to the variability in filling time caused by the irregular rhythm that will not reflect volume responsiveness. Simply, there can be tremendous stroke volume and pulse pressure variability with a significant dysrythmia that is related to the rhythm problem itself and not volume status. In these instances, stroke volume variation or pulse pressure variation tells us nothing about volume responsiveness or the patient’s position on the Frank-Starling curve. Similarly, albeit through a different mechanism, an irregular breathing pattern, i.e. spontaneous breathing commonly, where both the tidal volume and the cycle length from breath to breath vary, may also induce stroke volume variation that is not related to volume responsiveness. The more irregular the breathing pattern and depth of ventilation is, at least potentially the more exaggerated this problem becomes. This is further complicated by insufficient pleural pressure change in spontaneously breathing patients to meaningfully impact venous return. The use of dynamic variables for volume responsiveness has not and will not be validated generally in spontaneously breathing patients for these reasons.
The question then becomes what to do with these patients to ascertain whether or not they are volume responsive. Fortunately, the answer is simple and intuitive as long as we have the ability to measure a change in stroke volume. This represents a huge advantage of the technologies that allow measurement of stroke volume and cardiac output, namely FloTrac/Vigileo, PICCO, and LIDCO, over those that simply provide pulse pressure variation, which is now easily available on bedside monitors that display an arterial waveform. This physiology also explains why the bulk of this literature has been developed in the operating room where the ideal conditions often exist for the measurement and application of dynamic parameters, namely controlled mechanical ventilation with a large enough tidal volume to induce a significant change in pleural pressure to impact venous return. Indeed, a threshold value for tidal volume of 8 cc per kilogram ideal body weight has been determined in several studies that evaluated this specific question, the concept being that below this threshold value of 8 cc/kg, there is not enough pleural pressure change to meaningfully impact venous return. Across a heterogeneous population, this may indeed be true, although there will be specific examples depending on the patient’s lung compliance and intravascular volume status where it may not be. At lower tidal volumes, you may get some false negatives, for lack of a better term, where stroke volume variation is low, but the patient may still be significantly volume responsive. The converse is unlikely to be true. If at a lower tidal volume, there is significant variability in stroke volume, generally those patients would be expected to be volume responsive. We encountered extreme examples of this during the H1N1 flu epidemic in the fall of 2009, where many of our patients were being oscillated at very high frequencies but with minimal tidal volume and hence pleural pressure change. These patients could be significantly volume responsive with essentially no stroke volume or pulse pressure variation because of the imperceptible change in pleural pressure with this strategy of ventilation. Similarly, patients being ventilated using a low tidal volume strategy may manifest a similar phenomenon. In those patients, teasing out volume responsiveness utilizing physiology is fairly simple. What we do in my Intensive Care Unit for these patients is one of three things:
We acutely increase the tidal volume to at least 8 – 10 cc/kg and look for the change in stroke volume variation. For volume responsive patients, once we go beyond the threshold value for tidal volume, often this becomes apparent in stroke volume variation, it will increase to greater than 13%. The time responsiveness of these technologies is fairly rapid and tidal volume needs to be increased for only a short period of time, less than 5 minutes with the FloTrac/Vigileo system and often 2 minutes is enough to show significant change. We will also stand at the bedside during this maneuver to a) view the change; and b) assure that the pressures encountered with the ventilation change are not harmful to the patient.
Another strategy that has been proven to be extremely effective in this regard is the passive leg raising maneuver (PLR). The patient is laying flat and the legs are elevated to 45 degrees (Figure 4).
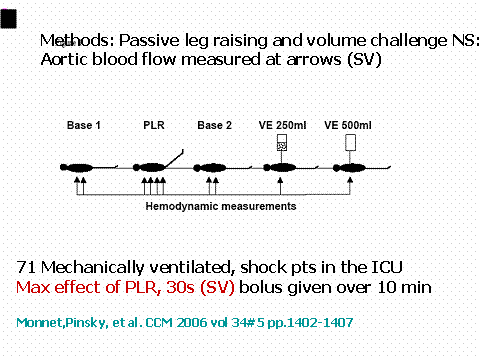
Figure 4
(Those patients in whom stroke volume variation becomes greater than 13% are then given a fluid bolus typically after being returned to the initial ventilator settings with the tidal volume approach, or returned to their prior position in bed after the PLR maneuver and the change in stroke volume and cardiac output is recorded. This can be done numerous times until the indicator of volume responsiveness disappears or an adequate stroke volume/cardiac output is reached.) The legs need to be elevated for roughly 120 seconds using the FloTrac/Vigileo system and a similar time for the PICCO system to assess what the increase in venous return from this gravitational maneuver induces in cardiac performance. Those patients that have a positive response usually defined as greater than 12-15% increase in cardiac output are then given a fluid challenge. In numerous studies, this has been shown to be essentially a perfect test as a measure of volume responsiveness as long as the improvement in stroke volume/cardiac output is the cardiac performance parameter of interest. This technique works well especially in those patients who are either spontaneously breathing or have a significant dysrhythmia. This reversible volume challenge is most useful in patients with acute lung injury or ARDS or those with either acute or chronic renal failure, where giving a volume challenge that does not result in improvement in cardiac performance may in fact be detrimental to the patient.
As long as there is a cardiac performance measure, again stroke volume or cardiac output, simply giving a fluid bolus and assessing its impact on cardiac performance is a reasonable way to go in those patients in whom we suspect that additional volume will not be injurious, i.e. clear lungs or without renal failure. If cardiac performance does not improve, volume is not the answer. When beginning to utilize these technologies, it is not uncommon to have to prove this to yourself several times, i.e. giving multiple fluid challenges and seeing no improvement in cardiac performance, but this is all part of the learning curve with new technologies.
At some point, however, it is important to recognize that the patient is on the flat part of the Frank-Starling Curve and not responding to volume (Figure 3), as ultimately excess volume has been associated with increased length of stay, increased time on mechanical ventilation, and mortality. The major impact of using these technologies well is that we now have the ability for precise titration of volume management in the majority of critically ill patients. A simple premise to consider as I close is why do we give volume or attempt volume challenges in the critically ill in any circumstance. My strong contention is the only reason we do this is to affect a change in cardiac performance, thus begging the question on how are we able to do this intelligently, using physiology, without a cardiac performance measure?
May , 2011 - Blog # 1 - Dr. McGee on Hemodynamics
William T. McGee, M.D., MHA, FCCP is Associate Professor of Medicine and Surgery at Tufts University School of Medicine, Boston, Massachusetts. His interests are in ARDS, vascular access (pulmonary artery catheterization), sepsis, nutrition, and nosocomial pneumonia. He has published > 67 papers, chapters and abstracts. He is the principal investigator for clinical trials studying the efficacy and safety of rfPAF-AH for the prevention of ARDS in patients with severe sepsis. He is a three-time recipient of Excellence in Teaching Award from Tufts University School of Medicine, the Society of Critical Care Medicine Internal Medicine Specialty Award for “Influence of Insurance Status on Pulmonary Artery Catheter Use” and The Presidential Citation Award from the Society of Critical Care Medicine for outstanding contribution to the Society during 1999. He is a Director for the Fundamentals of Critical Care Support Course for the Society of Critical Care Medicine. He is on the review board for the Journal of Intensive Care Medicine.

Your feedback is important to us. Please Contact Us to let us know how to make this site better. Thank you.